Agriculture
We are actively working to develop new fertilizer additives to improve the growth and harvest yields of crops grown in the U.S. We are using our knowledge of organic chemistry along with fundamental knowledge of how gasotransmitters and fertilizers function in plants to develop a next generation of fertilizers. The goal is to increase the survival of crops to heat and drought stress that is increasing with global warming while increasing their harvests. We are taking a chemical approach to this problem by using signaling chemicals in plants to tell them to grow bigger, faster, stronger. We are pursuing this work with Professor Erin Irish in the Deparment of Biology and Professor Aliasger Salem in the College of Pharmacy.
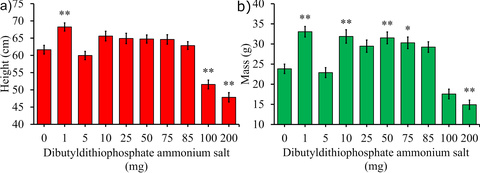
Carter, J. M., Brown, E. M., Irish, E. E., Bowden, N. B. Characterization of dialkyldithiophosphates as slow hydrogen sulfide releasing chemicals and their effect on the growth of maize. Journal of Agriculture and Food Chemistry, 2019, 67, 11883-11892.
Carter, J. M., Brown, E. M., Grace, J. P., Irish, E. E., Bowden, N. B. Improved growth of pea, lettuce, and radish plants using the slow release of hydrogen sulfide from GYY-4137. PLoS ONE, 2018, 13, e0208732.
Polymers for drug or gene delivery
We have a project to develop new methods and polymers for the delivery of drugs or genes to selected cells to treat a variety of diseases and cancer. In the fields of drug and nucleic acid-based therapies, a nanoparticle with a typical size of one hundred nanometers is fabricated from a biodegradable polymer loaded with drugs or nucleic acids that are noncovalently encapsulated within the particle. The polymer protects the drugs or nucleic acids from degradation in the bloodstream and allows their delivery to tumors where they are taken into cancer cells. The polymers used in this field degrade slowly in the blood stream but have a rapid rate of degradation when taken into the acidic compartments of cells - the endosome and lysosome - where they release their cargo. It is critically important that the polymer be biodegradable such that it will not accumulate within the body and cause a toxic response. Polyesters are the most common type of polymer used in these fields because the ester bond degrades slowly in the bloodstream but rapidly degrades under acidic conditions.
We recently developed new polymers based on bonds between sulfur and nitrogen that possess many of the right characteristics for drug/gene delivery. These polymers are stable in organic solvents, slowly degrade in water under neutral conditions, are nontoxic, and rapidly degrade in water under acidic conditions. We are the first to synthesize polysulfenamides, polydiaminosulfides, and polydisulfidediamines. We are currently investigating potential applications of these polymers in medicine and other fields.
We are actively applying these polymers in drug/gene delivery in collaboration with Professor Aliasger K. Salem in the College of Pharmacy at the University of Iowa.
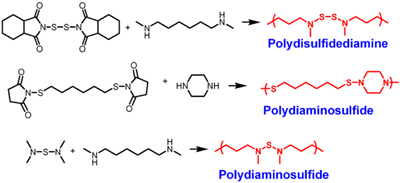
Graf, T. A.; Yoo, J.; Brummett, A. B.; Lin, R.; Wohlgenannt, M.; Quinn, D.; Bowden, N. B. “New polymers possessing a disulfide bond in a unique environment” Macromolecules, 2012, 45, 8193-8200.
Note: A review of this article and the following two appeared in Chemical & Engineering News. See Chemical & Engineering News, 2012, 90, issue 35.
Yoo, J.; Kuruvilla, D. J.; D’Mello, S. R.; Salem, A. K.; Bowden, N. B. “New class of biodegradable polymers formed from reactions of an inorganic functional group” Macromolecules, 2012, 45, 2292-2300.
Note: This article was reviewed in Synfacts. Synfacts is a journal to describe important trends and results in organic chemistry. Swager, T. M.; Mirica, K. A. “First synthesis of polysulfenamidies” Synfacts, 2012, 8, 611.
Yoo, J.; D’Mello, S. R.; Graf, T.; Salem, A. K.; Bowden, N. B. “Synthesis of the first poly(diaminosulfide)s and an investigation of their applications as drug delivery vehicles” Macromolecules, 2012, 45, 688-697.
Size-selective membranes based on polydicyclopentadiene for the filtration of organic molecules
We recently discovered that membranes composed of polydicyclopentadiene separate organic molecules with molecular weights from 100 to 1,000 g mol-1 based on their cross-sectional areas without regard to their molecular weights. For instance, tributylamine and triisobutylamine both have the same molecular formula but differ in their arrangement of atoms in space. Triisobutylamine (cross-sectional area: 0.38 nm2) permeates through the membranes over four orders of magnitude faster than tributylamine (cross-sectional area: 0.50 nm2). No other polymeric membrane can separate these isomers.
This result is exciting because many common ligands for metals have large cross-sectional areas and will not permeate the membranes. Thus, our membranes allow the products of a reaction to permeate but retain the ligands and metals. Our membranes can be used to purify the product of a reaction and recycle the catalyst. This project is a continuation of our use of membranes fabricated from polydimethylsiloxane to site-isolate catalysts and reagents from one another in sequential reactions.
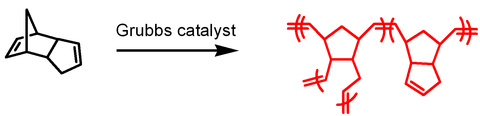
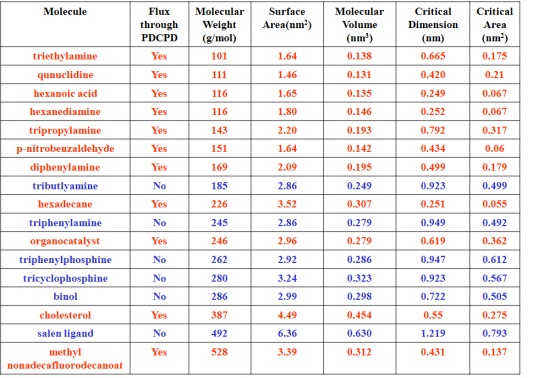
Gupta, A.; Rethwisch, D. G.; Bowden, N. B. “Site-isolation of palladium and phosphine ligands using nanoporous polydicyclopentadiene membranes” Chemical Communications, 2011, 46, 10236-10238.
Long, T. R.; Gupta, A.; Miler II, A. L.; Rethwisch, D. G.; Bowden, N. B. “Selective flux of organic liquids and solids using nanoporous membranes of polydicyclopentadiene” J. Materials Chem. 2011, 21, 14265-14276.
Miller II, A. L.; Bowden, N. B. “Simple site-isolation of up to >99.998% of PdCl2 and its recycling with PDMS thimbles” J. Org. Chem. 2009, 74, 4834-4840.
Mwangi, M. T.; Schulz, M. D.; Ned B. Bowden “Cascade Reactions with Grubbs’ Catalyst and AD-mix-a/b Using PDMS Thimbles” Organic Letters, 2009, 11, 33-36.
Miller II, A. L.; Bowden, N. B. “A Materials Approach to the Dual Site-Isolation of Catalysts Bonded to Linear Polymers and Small, Ionic Molecules for Use in One-Pot Cascade Reactions“ Advanced Materials, 2008, 20, 4195-4199.
Mwangi, M. T.; Runge, M. B.; Hoak, K. M.; Schulz, M. D.; Bowden, N. B. “A Materials Approach to Site-Isolation of Grubbs’ Catalysts from Incompatible Solvents and m-Chloroperoxybenzoic Acid” Chem. Eur. J. 2008, 14, 6780-6788.
Runge, M. B.; Mwangi, M. T.; Miller II, A. L.; Perring, M.; Bowden, N. B. “Cascade Reactions Using LiAlH4 and Grignard Reagents in the Presence of Water” Angew. Chem. Int. Ed., 2008, 47, 935-939.
Separation of fatty acids using size-selective membranes
We recently developed the first method to separate fatty acids from one another using a membrane. Over 150 million tons of vegetable oils are produced worldwide each year, but surprisingly few large scale applications of oils or their fatty acids have been developed. A reason for the lack of applications is that oils are composed of a triester with three fatty acids bonded to glycerol, and most oils are composed of five or more fatty acids. Thus, the oils have many different components that are very hard to purify. Furthermore, although it is easy to separate the fatty acids from glycerol, it is very challenging to separate each fatty acid from one another. Thus, any industrial application of fatty acids must use a mixture of fatty acids – and this is often not desirable.
We recently developed a very inexpensive method to separate fatty acids from one another by first complexing them with an amine and then separating them by filtration through membranes composed of polydicyclopentadiene. The fatty acid-amine salts can be separated from each other based on their different cross-sectional areas. This result is very exciting because it is the first method to purify individual components of fatty acids using a membrane. Separations based on membranes are inexpensive, so this method should allow the purification of fatty acids on a large scale that is impossible to do currently.
We have also expanded our research efforts in this area by developing new epoxy nanofiltration membranes that can separate out omega 3 fish oil ethyl esters, saturated fatty acids, and saturated fatty acid methyl esters. Omega 3 fish oil ethyl esters are important for heart health, and saturated fatty acid methyl esters are a crucial component of renewable fuels such as biodiesel. Membranes provide a greener separation technology than the industry standard for these separations, which is usually carried out by distillation.
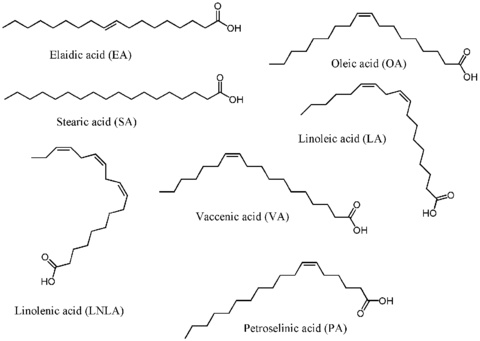
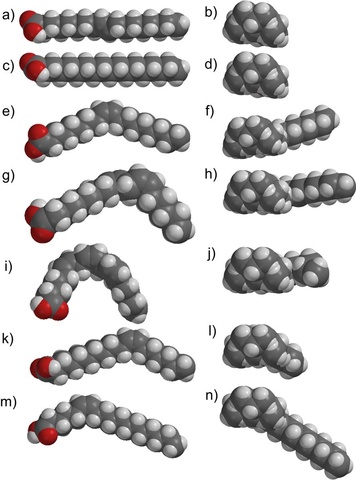
Gupta, A.; Bowden, N. B. “Separation of cis-fatty acids from saturated and trans-fatty acids by nanoporous polydicyclopentadiene membranes” ACS Appl. Mater. Interfaces, 2013, 5, 924-933.
Gilmer, C. M.; Bowden, N. B. "Highly-crosslinked Epoxy Nanofiltration Membranes for the Separation of Organic Chemicals and Fish Oil Ethyl Esters". ACS Appl. Mater. Interfaces. 2016, 8, 24104-24111.
Gilmer, C. M.; Zvokel, C.; Vick, A.; Bowden, N. B."Separation of Fatty Acids and Fatty Acid Methyl Esters with Epoxy Nanofiltration Membranes." RSC Advances. 2017, 7, 55626-55632.
Synthesis of polymer nanostructures
Nanoscience is one of the most studied areas of chemistry and holds much promise for the future. We developed the synthesis of an interesting class of organic nanoparticles called “comb block copolymers”. These polymers have a polymeric backbone with regularly and densely spaced polymeric arms. Because of steric crowding due to the polymeric arms, the backbones may assume a mostly extended conformation. Comb polymers possess shapes from spherical to cylindrical due to this steric crowding.
We synthesized some of the highest molecular weight comb block copolymers and studied their assembly in the solid state. These polymers assembled into ordered morphologies with domain sizes (i.e. the size of the repeat unit) up to over 200 nm. This is the largest domain size for block polymers without the use of an additive. These polymers were new photonic band gap materials and were colorful when viewed under light.
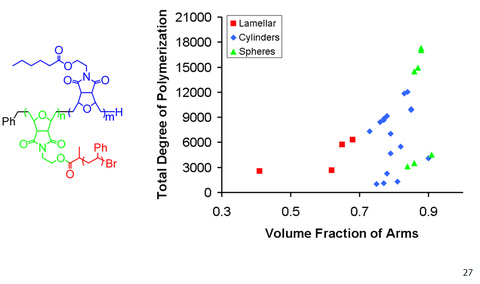
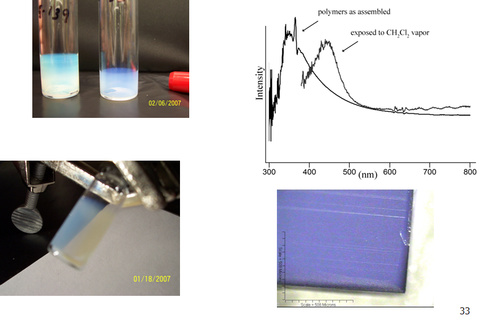
Yoo, J.; Runge, M. B.; Bowden, N. B. “Synthesis of complex architectures of comb block copolymers and their assembly in the solid state” Polymers, 2011, 52, 2499-2504.
Runge, M. B.; Yoo, J.; Bowden, N. B “Synthesis of Comb Tri- and Tetrablock Copolymers Catalyzed by the Grubbs First Generation Catalyst” Macromolecular Rapid Communications, 2009, 30, 1392-1398.
Runge, M. B.; Lipscomb, C. E.; Ditzler, L. R.; Mahanthappa, M. K.; Tivanski, A. V.; Bowden, N. B. “Investigation of the Assembly of Comb Block Copolymers in the Solid State” Macromolecules, 2008, 41, 7687-7694.
Runge, M. B.; Bowden, N. B. “Synthesis of High Molecular Weight Comb Block Copolymers and Their Assembly into Ordered Morphologies in the Solid State” J. Am. Chem. Soc., 2007, 129, 10551-10560.
Runge, M. B.; Dutta, S.; Bowden, N. B. “Synthesis of Comb Block Copolymers by ROMP, ATRP, and ROP and Their Assembly in the Solid State” Macromolecules, 2006, 39, 498-508.
Assembly of organic monolayers on polymers and Si(111)
In another area of nanoscience, we developed methods to assemble organic monolayers on directly on silicon and on polydicyclopentadiene. Silicon is the most important electronic material in use today, it is the basis for computer chips that are found in many common household items. Although there are many methods to assemble organic monolayers on silicon dioxide, there are relatively few methods to assemble organic monolayers directly on silicon. These monolayers are important because they may allow the electrical properties of silicon to be used in combination with atomic level control of the organic functional groups on the surface.
We developed a method to assemble ordered, organic monolayers on Si(111) using TEMPO. This method, originally developed by a talented undergraduate, is simple and passivates the surface from oxidation. We also developed methods to pattern these monolayers in the nanometer to micrometer size-range using PENs.
In related work, we developed two methods to assemble organic monolayers on polydicyclopentadiene. Polydicyclopentadiene is a very tough and stable polymer, but it possesses reactive olefins that can be functionalized. We developed methods to functionalize this polymer that may have broad applications in materials science.

Perring, M.; Long, T. R.; Bowden, N. B. “Epoxidation of the surface of polydicyclopentadiene for the self-assembly of organic monolayers” J. Mater. Chem. 2010, 20, 8679-8685.
Perring, M.; Bowden, N. B. “Assembly of Organic Monolayers on Polydicyclopentadiene” Langmuir, 2008, 24, 10480-10487.
Perring, M.; Mitchell, M.; Kenis, P. J. A.; Bowden, N. B. “Patterning by Etching at the Nanoscale (PENs) on Si(111) Through the Controlled Etching of PDMS” Chemistry of Materials, 2007, 19, 2903-2909.
Dutta, S.; Perring, M.; Barrett, S.; Mitchell, M.; Kenis, P. J. A.; Bowden, N. B. ”Cross Metathesis on Olefin-Terminated Monolayers on Si(111) Using the Grubbs’ Catalyst” Langmuir, 2006, 22, 2146-2155.
Arafat, S., Dutta, S.; Perring, M.; Mitchell, M.; Kenis, P. J. A.; Bowden, N. B. “Mild Methods to Assemble and Pattern Organic Monolayers on Hydrogen-Terminated Si(111)”, Chemical Communications, 2005, 3198-3200.
Perring, M.; Dutta, S.; Arafat, S.; Mitchell, M.; Kenis, P. J. A.; Bowden, N. B. ”Simple Methods for the Direct Assembly, Functionalization, and Patterning of Acid-Terminated Monolayers on Si(111)”, Langmuir, 2005, 21, 10537-10544.